Overactive bladder is a topic most people shy away from. We shouldn’t. Why? Because many of us will suffer from this inconvenient condition when we’re older. While there are treatment options and stop gaps like adult undergarments and continence hygiene products, they’re not perfect. Boston-based Australis Scientific led by CEO Nick Agahari in partnership with co-founder Martin Jensen are developing an at home treatment called the In-Confidence Smart Patch. This device reduces the symptoms of overactive bladder and is an alternative to other treatment options. Nick and Marty are Harvard Health Tech Fellows with backgrounds in Urology. Their understanding of the market and human issue is deep, and their technology is on target. The VisionTech Screening Committee was impressed so we invited Nick to present at our July virtual pitch events. Here’s a preview.

BP: How did you learn about VisionTech Angels?
NA: It’s kind of a funny story. One of your investors, Moa Feldenheimer, was participating in an MIT Bootcamp with a startup he’s working on. I was Moa’s coach and his team won the bootcamp! Moa rewarded me with an introduction to Vision Tech.
BP: Can you explain overactive bladder and who it affects?
NA: Sure! Overactive bladder, or OAB, is one of those conditions that becomes more prevalent as we age. OAB is predominantly idiopathic which means that there is an absence of pathologic or metabolic factors known to cause it. What happens is as we age, we become more prone to having inappropriate signaling to the brain that the bladder is full when it’s not. This causes “FUN” symptoms: frequency of urination, urgency to urinate, and nocturia or needing to urinate multiple times at night. Eighty percent of people aged 75 have OAB and I assure you the FUN symptoms are no fun for patients.
BP: Tell me your quest to solve the issue.
NA: My co-founder Martin Jensen and I met through the Harvard HealthTech Followship in 2021. Only four fellows are chosen each year from hundreds of applicants from around the world, so it’s a high honor. We were embedded for three months at Harvard’s Brigham and Women’s Hospital Department of Surgery to identify unmet clinical needs. What we identified was that the FUN symptoms were pervasive among the patients treated by urological surgeons. Over 90% of patients we observed received surgery, minimally invasive therapies, and medical management to manage their lower urinary tract symptoms which include overactive bladder. Current treatment options weren’t satisfactory for patients or physicians. Ultimately, this discovery set us on the path to create a solution.

We did a lot of patient and physician interviews and discovered most patients diagnosed with OAB are managed with prescription medicine. Those patients who are not responding to medication have more advanced invasive procedures like Botox injections in their bladders and surgery to implant a lead and pacemakers into their tail bone to control the urge to urinate. We heard a lot of frustration in our interviews. It’s a difficult decision for patients to either take medications which come with adverse side effects or commit to invasive interventions like surgery.
Both Marty and I could personally relate to them. Marty cared for his elderly father-in-law who had Alzheimer’s. Having a better solution that would help him sleep through the night and reduce the number of diapers would have made his care easier and his last years more dignified. My own grandfather had OAB and was very traumatized by it. He tried to be stoic, but it drove him to tears because it robbed him of his independence and identity. This was a man who was a POW during World War II.
BP: What was the unmet need that you identified?
NA: People become very frustrated with the indignity of wearing adult diapers to manage their hygiene. They become noncompliant with the medications due to the adverse side effects and the increased risk of dementia. The advanced therapies such as surgery and injections are often not an option especially in the elderly patient cohort. The majority of OAB patients suffer in silence and undiagnosed due embarrassment and the misconception that their urinary symptoms are a normal part of aging.
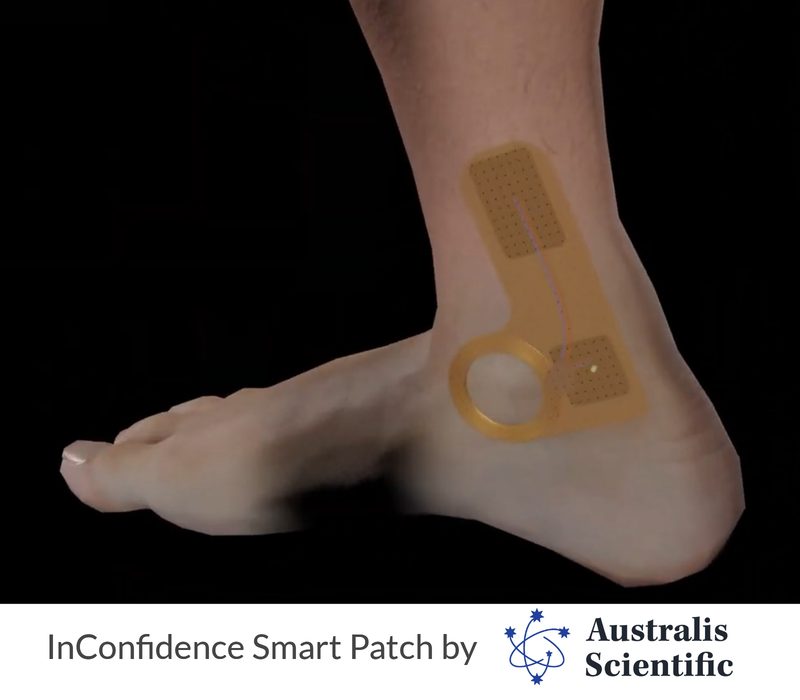
BP: What is the InConfidence Smart Patch and how does it work?
NA: Our goal with the In-Confidence Smart Patch is to develop a novel solution to treat patients with overactive bladder that is discreet, effective, and easy to use without requiring any major lifestyle modifications.
In-Confidence Smart Patch is a minimally invasive and does not require anesthesia, surgical incision, cystoscopy, or deep needles to deliver treatment. Basically, it’s a “set and forget” smart patch worn on the ankle with microarray needle electrode technology. This technology stimulates the posterior tibial nerve in the ankle to relieve the symptoms of OAB. In-Confidence is also capable of delivering maintenance therapy to minimize overall FUN symptoms. Patients activate on-demand therapy to mitigate urgency symptoms and automated on-time therapy to minimize symptoms of nocturia. Patients require a new smart patch each week.
BP: Why do you think people will find it more palatable than adult diapers, medications and surgical intervention?
NA: One of the biggest advantages of our technology is ease of use. It’s almost like wearing a sticker. If the patient has ever worn a patch for smoking cessation or diabetes, they will be comfortable wearing our patch. It’s noninvasive and doesn’t require any behavior modifications. The patient literally puts on the patch once a week and forgets it.
Compare this with adult diapers, which are bulky, embarrassing, and don’t fit with the active lifestyles many patients want to regain. Medications, while easy to use, have a lot of negatives. They can make patients feel mentally foggy and because these meds dry up a person’s orifices—the mouth for example—they feel the urge to drink more which stimulates urine. OAB medication have also been proven to increase the risk of dementia and that’s frightening for people and concerning to clinicians. Surgical intervention isn’t always the best option for seniors largely due to the risk of anesthesia and being invasive to the body.

BP: How big is the addressable market in the United States?
NA: OAB is an enormous problem, particularly among older adults. In the United States alone, 40 million people have FUN symptoms. Currently, only about 16 million seek clinical diagnosis and treatment. Of these, about 6.8 million are resistant to medication and would benefit from percutaneous tibial nerve stimulation. We are focusing first on the 65,000 patients per year who are receiving neuro-stimulation interventions through their urologist, a segment is $228 million annually in the U.S.
BP: Your product requires a physician prescription. Will physician adoption be a challenge?
NA: The In-Confidence Smart Patch is a prescription only device. It’s currently considered as a third-line therapy by the American Urological Association and prescribed after the patient can no longer tolerate prescription medications. As we build our clinical efficacy and health economic data, we hope to be reclassified as a second-line device so patients have an alternative to medication therapy.
In terms of physician adoption, OAB patients are treated by urologists, a subspecialty that’s in high demand. These patients are time-consuming to treat and generate low revenue. During our physician interviews, the response was positive because of favorable outcomes and patients require less hands-on time to manage. A non-pharmaceutical, non-invasive treatment option is also very beneficial for this generally older patient population. Finally, we fit directly into established reimbursement codes, so physicians are compensated while freeing time to increase patient volumes.

BP: Do you have any patents on your technology?
NA: We have patents in the national phase and provision applications. We expect our foundational patent to be granted in the next 12 months. We have disclosed further patent applications through Harvard Office of Technology developed as part of our fellowship, which we intend to license. It’s worth noting that Harvard creates quite a moat; they do not hesitate in litigating any patent infringements.
BP: Explain your revenue model.
NA: The InConfidence Smart Patch has two components: an induction powering unit that is a one-time purchase and the consumable patches, which are sold as a weekly subscription. We are leveraging existing CPT and ICD codes for percutaneous tibial nerve stimulations, which covers physician fees and device reimbursement.
BP: Where are you in terms of commercialization?
NA: We have finished our preliminary development cycle and are finalizing our prototype. We have partnered with a development, design, and engineering firm with roots at MIT and experience in bringing medical devices to market. We have implemented a world class quality management system, and are working toward design lock and filing a 510(K) pathway for a class II medical device with the FDA.

BP: What’s your go-to-market strategy?
NA: We are looking to a market launch in Q4 2024. Our beach head will likely be The Villages in Florida, a retirement community with about 130,000 residents. The median age is 72 and about 95% are covered by Medicare. There are 20 urologists serving the community who will be our call points. Market development will be led by a sales team experienced in Urology.
BP: What funding round is this?
NA: This is our seed round.
BP: What is your planned use of funds?
NA: We are very focused on finalizing our prototype, expanding intellectual property, identifying manufacturing partners and securing FDA approval.
BP: Give three reasons why VisionTech Angels should invest.
NA: First, there is a significant unmet medical need around the world, which we have validated. We have identified a market of 33.2 million Americans looking for a discreet treatment that In-Confidence offers. Second, as Harvard Health Tech Fellows , we have been focused on our technology and strategy for the last 12 months with access to incredible resources at Harvard. Lastly, our founding team has deep experience and expertise in Urology, empathy for this patient population, and direct experience with medical devices and building sales teams for global companies such as Boston Scientific and Medtronic. We believe it’s a winning combination.

VisionTech Angels’ July Pitch Events will be virtual on Thursday, July 28 at Noon ET and at 6 p.m. ET. Pitch events are open to our members and accredited investors interested in joining our group. To register, check your email for an invitation, go to our Events page where you’ll find the RSVP links, or email Ben Pidgeon at bpidgeon@visiontech-partners.com.
