Last year was a very good year for VisionTech. Applying strict criteria, we completed 18 deals worth $2.88 million. We’re continuing our thesis this year, looking hard at leadership, the unmet needs being met, milestones achieved, and deal terms. We’re also leaning into syndication partners for deal flow and diligence. Early this year, New World Angels of Boca Raton, Florida, suggested we look at a pioneering biotech startup called Aegle Therapeutics. Last year, both of our groups invested in NuvOx Therapeutics, so I was open to reviewing Aegle and its novel platform therapy for severe burns and other rare and challenging skin conditions.Impressed with CEO Shelley Hartman, her “EV” technology’s potential impact on lives and traction, I invited her to present at our February 29 pitch events. Here’s a preview.

BP: Before we get started, I heard you are the proud mom of a former high school football player.
SH: (Smiles) That would be my daughter Sofia. During her senior year in high school, she was a starting running back for the boys’ varsity team. Before the season started, the coach took me aside and said, ” Do you know why she is so good? She can see the hole and run through it.” And so she did. All season.
BP: That’s a great story. Your background is in banking. How did you get involved with a biotech startup out of the University of Miami?
SH: You must be reading my LinkedIn profile, Ben. Yes, I spent nearly 20 years with First Boston and Goldman Sachs. In both cases, my focus was life sciences and healthcare services companies: advising, raising capital, mergers and acquisitions. In 2004, I was recruited to Fort Lauderdale, Florida, to run LifeSync Holdings, a corporate incubator developing biopharma, medtech and diagnostic products. We were funded by TGP, Medtronic, 3M, and other large investors; it was a great experience. I wrapped up that role in 2013, but because of my daughter’s football, weightlifting and lacrosse career, I wanted to stay in Florida. So I became an entrepreneur-in-residence (EIR) for the University of Miami Miller School of Medicine.
BP: How did you get involved with Aegle Therapeutics?
SH: I was introduced to Bob Williamson in the tech transfer office at the University of Miami Miller School of Medicine. He’s a serial life sciences entrepreneur and was looking at licensing some technology around stem cells and wanted my help on it. That tech came from the lab of Dr. Van Badiavas. Van had found a way to harness the poer of stem cells without using the cells. This was the foundation of what would soon become Aegle Therapeutics. I reviewed it and thought the science was brilliant and very elegant. We ended up licensing the technology and I came onboard with Aegle full-time in 2019 as CEO.
BP: You mentioned something called “EVs” in an earlier conversation and all I could think about was Tesla. Can you explain, in layman’s terms, what you’re doing with Aegle and EVs.
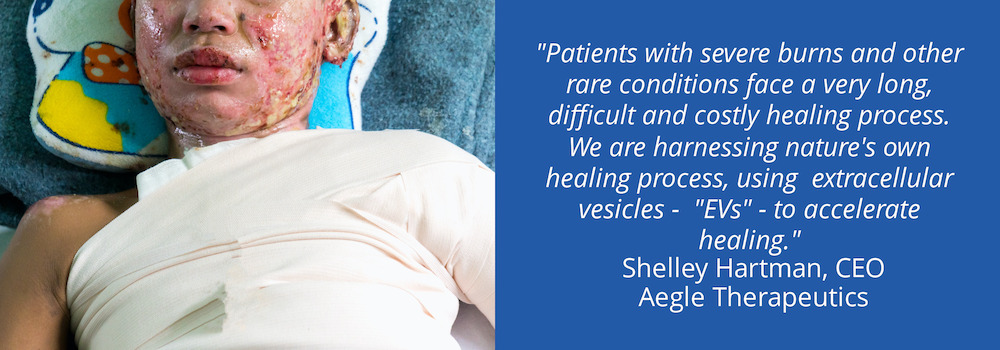
SH: The science does get pretty deep, but here’s the elevator version: Aegle is developing novel, extracellular vesicle (EVs) therapies in the form of a topical medication to treat rare and severe dermatological disorders with significant unmet medical need. Our initial targets are severe second degree burns and a rare pediatric, skin condition called dystrophic epidermolysis bullosa.
You’ve probably heard of stem cells being used to treat cancer and other diseases. Well, we are taking EVs, which are secreted by stem cells, and using them to influence the immune system, accelerate healing, support blood vessel growth and neuronal regeneration, and minimize inflammation and scarring . Using EVs, we are harnessing the body’s own power to heal itself faster and more completely.
BP: Share an example.
SH: Think about someone who’s experienced severe burns in a fire, a work or recreational accident or on a battlefield. Burn wounds are extremely difficult for patients and physicians. They’re painful, they swell, they’re slow to heal, and cause terrible scarring. It can cost millions per patient to treat. If skin grafts are required, that’s another layer of complexity, pain and cost. We recently treated our first patient whose foot was charred in a boating accident. Withing seven days of one dose of our EV-based topical, his burn wound was closed, there was a significant reduction in swelling, and no sign of ischemic reperfusion injury. In four weeks, his pain was gone, and in 12 weeks, his foot was completed healed.
BP: That’s impressive! I can see why the military would be interested in this.
SH: They are! We have $1.5 million in non-dilutive funding through the Congressionally Directed Medical Research Program, specifically for biotech innovations like ours.
BP: Why hasn’t this been addressed before?
SH: Our overall approach is novel, but it’s our manufacturing approach that truly differentiates our platform. Our lead product, AGLE-102™, is a natural composite of EVs; it’s not engineered. Our method of isolating and collecting the EVs is very precise, safe, and does not damage or modify the EVs. The end product mimics the body’s own natural production.
BP: We always want to know about IP to ensure companies’ moats are deep and wide.
SH: We’ve definitely got that covered. We have 85 patents of which 55 have been granted. Many of these are around our manufacturing and composition of matter. Our patents cover all major markets, including the United States, EU, Japan, Australia, and Canada. We’ll continue adding to our IP as we add to our pipeline.
BP: What round is this?
SH: This is a $5 million Series A preferred stock round. Right now we have commitments for $2.8 million and would like to close on $3 million by the end of February.
BP: What’s the planned use of funds?
SH: Basically to continue our momentum. We plan to use the proceeds to generate strong clinical data in both our burn and dystrophic EB clinical trials, which we hope shows AGLE-102 as a new modality to treat other inflammatory and immune-based dermatologic disorders.
BP: Give me three reasons why VisionTech Angels should invest in Aegle.
SH: First, it’s the perfect time to get behind our company. We recently completed the proof of concept in our burn trial and the results exceeded expectations. We are moving forward with our second clinical trial, dystrophic EB, which is a major inflection point. Third, our manufacturing process is unique, challenging and the IP behind it is extensively protected. We have successfully completed multiple GMP manufacturing runs. Here’s a fourth reason: we all know it’s a challenging time for biotech startups to be fundraising. We’d like to close the round quickly, so our pre-money valuation is very favorable to investors.
VisionTech Angels’ Virtual Pitch Events will be held Thursday, February 29 at 12 Noon and 6 p.m. ET. Pitch events are open to our members and accredited investors interested in joining our group. To register, check your email for an invitation, go to our Events page where you’ll find the RSVP links. You can also email Ben Pidgeon at bpidgeon@visiontech-partners.com.