
VisionTech Angels’ Executive Director Ben Pidgeon recently sat down with David Winternheimer, co-founder and CEO of Glutenostics, to learn more about how his company is redefining how those with celiac are diagnosed and manage their disease. David will be presenting Glutenostics during our first Pitch Week of 2019, February 25-28.
BP: What is the current state of celiac disease in the U.S. and how is it diagnosed?
DW: Celiac disease is a serious autoimmune disease triggered by ingesting gluten that damages the villi of the small intestine and interferes with absorption of nutrients from food. More than 1% of the U.S. population—about 3.5 million people—have celiac disease. Of those individuals, 85% have yet to be diagnosed. As many as 10% of the population are gluten intolerant and have painful symptoms after eating products containing wheat, barley or rye. The standard test for diagnosing celiac disease involves an endoscopy and biopsy and requires eating gluten daily for four to six weeks before the procedure. Many patients seeking diagnosis are already gluten-free, so this ‘gluten challenge’ is a major barrier to diagnosis. Our new blood test circumvents the need to eat gluten in order to get a diagnosis, and is much easier than a biopsy. Additionally, there has been no way to monitor compliance with a gluten-free diet.

It’s also worth noting that access to diagnostics and ongoing care for this condition is highly lacking. Family doctors and internists don’t do biopsies to diagnose celiac disease and gastroenterologists don’t have a way to diagnose patients who are gluten free and refuse to reintroduce gluten into their diet. There are; however, about 1,200 celiac physicians in the United States and about 200 celiac clinics nationwide. Glutenostics’ technologies were developed over the past decade in response to thought leaders’ explicit call for better tools to diagnose and manage the disease.
BP: What solutions does Glutenostics offer?
DW: Glutenostics was founded in 2016 with the mission of bringing new technologies to market that improve the diagnosis, monitoring, and quality of life of those with celiac disease and gluten intolerance. In 2017, we launched our first product line, Gluten Detective, a rapid at-home urine and stool monitoring test, that’s much like a pregnancy test, to measure compliance with a gluten-free diet.
We’re now preparing to launch our lab tests, including a blood flow cytometry test for diagnosing celiac disease that involves a proprietary HLA gluten tetramer reagent and doesn’t require the eating gluten prior to the test. We also plan to launch a lab version of the urine/stool test for diet monitoring as well for physicians to order We’re working with multiple collaborators nationwide at major institutions to drive the adoption of both of these technologies into the official celiac clinical guidelines. The Harvard celiac program continues to be our biggest advocates.
BP: Is this technology you developed or licensed? Is it patent protected?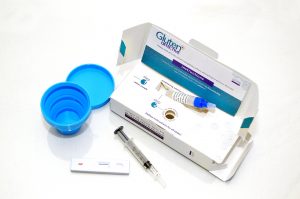
DW: Glutenostics has exclusive licensing rights for both the blood diagnostic and urine/stool monitoring tests in the U.S. and Canada. The blood test comes from Ludvig Sollid’s lab at the University of Oslo, Norway, a world-renowned immunology lab that is well respected among the celiac community. The urine and stool tests comes from Biomedal of Seville, Spain, whose CEO is also a co-founding member of Glutenostics and credited with developing the world’s second most commonly used test for assessing gluten content in foodstuffs.
BP: What are the regulatory and reimbursement requirements?
DW: As lab developed tests, the FDA does not regulate tests such as our flow cytometry and at-home rapid tests. CMS currently reimburses similar blood tests at a rate of about $400 and private payers reimburse at around $1,000, which is a third of the cost of a biopsy, hence the appeal to payers. We don’t yet have reimbursement rates yet for the at-home rapid test, but we’re working on that.
BP: Does Glutenostics have an Indiana connection?
DW: I’m a native of Evansville and our CLIA lab partner, Xeno Diagnostics, is located in Indianapolis, where we’re in the process of establishing our blood diagnostic test as a CLIA Lab Developed Test. Immediately after closing this round of financing, we will move our distribution center for the at-home kits to Indy as well as establish our physical headquarters office here, too.
BP: What kind of patient advocacy support do you have?
DW: The celiac community is extremely enthusiastic and supportive about what we’re doing. We have partnerships with Beyond Celiac and the Celiac Disease Foundation, which are both trusted U.S. non-profit groups for celiac disease. By partnering with these organizations, we will work to educate the celiac community about our diagnostic test and at-home testing product, Gluten Detective. We also have the support of all major celiac centers and key opinion leaders nationwide.
 BP: Do you have customers and are you generating revenue?
BP: Do you have customers and are you generating revenue?
DW: We are currently generating revenue from our direct-to-consumer at-home monitoring test, Gluten Detective. We have a clear plan to drive future sales and clinical adoption of our new tests.
BP: Looking forward to your pitch!
DW: We’re looking forward to the road show!
To learn more about Glutenostics, visit their website. For details on VisionTech Angels’ February Pitch Week, visit our events calendar.